Covalent Bonding
What you need to know:
What is covalent bonding
How electrons are shared in covalent bonding
Covalent bonding is the type of bond that forms between nonmetals and nonmetals. Covalent bonding is when two (or more) nonmetals share electrons to acquire a full outer shell of electrons.
For example:
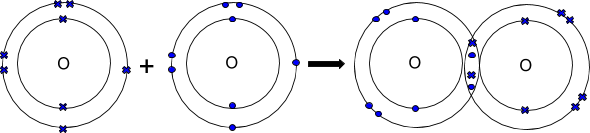
Oxygen atoms prefer to travel around with another oxygen atom. This is because both Oxygen atoms have 6 electrons in their outer shells. When the two oxygen atoms form a covalent bond they each share 2 electrons which means that they both now have 8 electrons in their outer shells.
A few things to remember:
- Covalent bonding happens between nonmetals and nonmetals
- Neither atom becomes charged
- The atoms share electrons with each other to fill their outer shells.
Representing Covalent Bonding
What you need to know:
How to draw a dot cross diagram for covalent bonding.
The different ways in which bonds can be represented.
Dot Cross Diagrams



Here are the same 3 covalent substances: Ammonia, Oxygen and Hydrogen, as dot-cross diagrams. If you are asked to just show the covalent substance as a dot-cross diagram, just show the final product! Notice how there can be more than one shared pair of electrons! Each shared pair is a covalent bond (so the more shared pairs, the stronger the bonding).
Pros of dot-cross diagrams:
- Can easily see where the electrons have come from
- Easy to draw
Cons:
- Don’t show the relative sizes of the atoms
- Can’t see how the atoms are arranged in space
Displayed Formulas

Here are the same 3 covalent substances, Ammonia, Oxygen and Hydrogen, as displayed form. Notice how each line between the atoms (bonds) represents a shared pair of electrons (so Oxygen is sharing 2 pairs and has two lines connecting the two atoms).
Pros of displayed formula form:
- Quick and easy to draw (so useful for large molecules)
Cons:
- Can’t see how the atoms are arranged in space
- Can’t see where the shared electrons have come from
3D models and ball-stick models

Here are the same 3 covalent substances, Ammonia, Oxygen and Hydrogen, as 3D stick and ball models.
Pros of 3D stick and ball models:
- hey show the arrangement of molecules in space
Cons:
- difficult to draw and easily get confusing
- don’t know where the electrons have come from
Simple Molecular Substances
What you need to know:
Learn and remember the covalent bonding dot cross diagrams of 8 types of simple molecular structures.
You will need to know the bonding in the following simple covalent substances:
- Hydrogen H2
- Oxygen O2
- Ammonia NH3
- Chlorine Cl2
- Hydrogen Chloride HCl
- Methane CH4
- Water H2O
- Nitrogen N2
If you remember all of these, you will be fine is MOST questions. If you understand all of these, you’ll be fine!!!
Hydrogen, Oxygen and Ammonia were show in the last section. Here are the other 5!
1. Chlorine

2. Hydrogen Chloride HCl

3. Methane ____CH4

4. Water ____H2O

5. Nitrogen ____N2

Properties of Simple Molecular Substances
What you need to know:
How the different properties of simple covalent structure.
Understand what intermolecular forces are.
All simple molecular covalent substances share similar properties, such as electrical conductivity and melting/boiling points.
Electrical Conductivity
Electrical conductivity is just how much a substance lets electricity pass through it. You may know from physics, that the only way electricity can pass through something is if there are charged particles that are free to move around.
In covalent substances, there are no charged particles that are free to move around, therefore they do not conduct electricity!
Intermolecular Forces
When something is melted or boiled, the molecules are separated from one another. For example, if you had a bowl of water (lots of H2O molecules) and you boil the water, the H2O molecules will all separate from one another. DO NOT get this confused with separating the atoms. The molecules are still intact.
The different molecules in a liquid or solid are attracted to each other via intermolecular forces. This is what keeps them all bundles up (unlike a gas).
When something is heated, the heat breaks these intermolecular forces. The __larger the molecules, the more intermolecular forces __and the __more heat is needed __to break all of the intermolecular forces. Therefore, small molecular substances have low melting/boiling points (need little heat to break the intermolecular forces) and larger molecular substances have high melting/boiling points (need lots of heat to break all of the intermolecular forces).

_Note: Intermolecular forces are NOT the same as bonds. _
- Bonds are things that keep atoms together to make molecules.
- Intermolecular forces are forces of attraction that keep molecules close together (but they’re still free to move and not bonded to one another.
Melting and boiling points of simple covalent substances
Since all simple molecular substances are composed of small molecules, they don’t require much heat to break all of the intermolecular forces. This means that they have LOW boiling and melting points!
Trends in melting and boiling points of simple covalent substances
As the molecules get bigger, the amount of intermolecular forces to overcome increases (melting and boiling points increase). The size of a molecule doesn’t only depend on how many molecules there are, but how big the atoms are too!

You may remember from the PeriodicTable section, that the size of an atom depends on how many electron shells there are, and how many protons there are in the nucleus. Let’s do a recap just to make sure!
Recap:
__The more electron shells, the larger the atom: __

So Na2 will have a higher melting point and boiling point than H2. This is because __Na2 __is larger, will have more intermolecular forces holding each __Na2 __molecule together and will therefore need more heat to separate all of the __Na2 __molecules from one another.