Brownian Motion
Explanation and evidence for Brownian motion
- An example of Brownian motion is the observed jerky and erratic motion of smoke particles as they are hit by the unseen molecules in the air which can be seen under a microscope
- In 1905, physicist Albert Einstein explained that pollen grains in water were being moved by individual water molecules
- In all cases, larger and visible particles are caused to move by the random bombardment of smaller, invisible particles
Extended Only
Diffusion & Molecular Mass
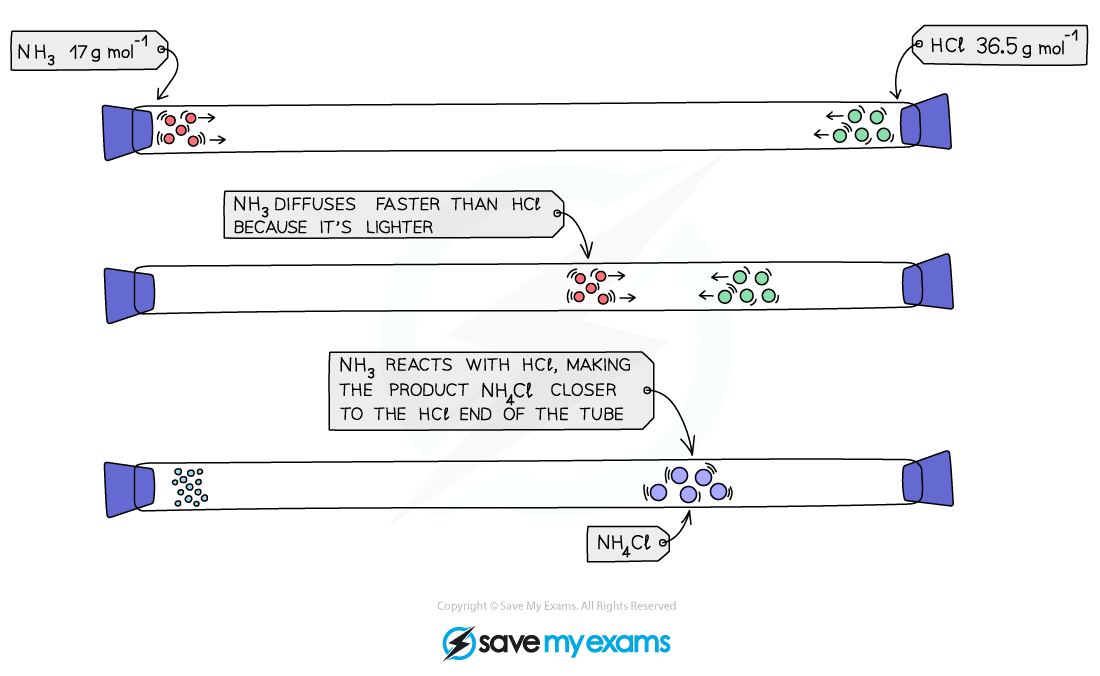
- Diffusion occurs much faster in gases than in liquids as gaseous particles move much quicker than liquid particles
- At the same temperature, different gases do not diffuse at the same rate.
- This is due to the difference in their relative molecular masses
- Lighter gas particles can travel faster and hence further, therefore the lower its relative mass the faster a gas will diffuse
NH3 molecules have less mass than the HCl molecule, so diffuse faster, hence the product (a white cloud of NH4Cl) forms closer to the end where the HCl is