Exothermic & Endothermic Reactions
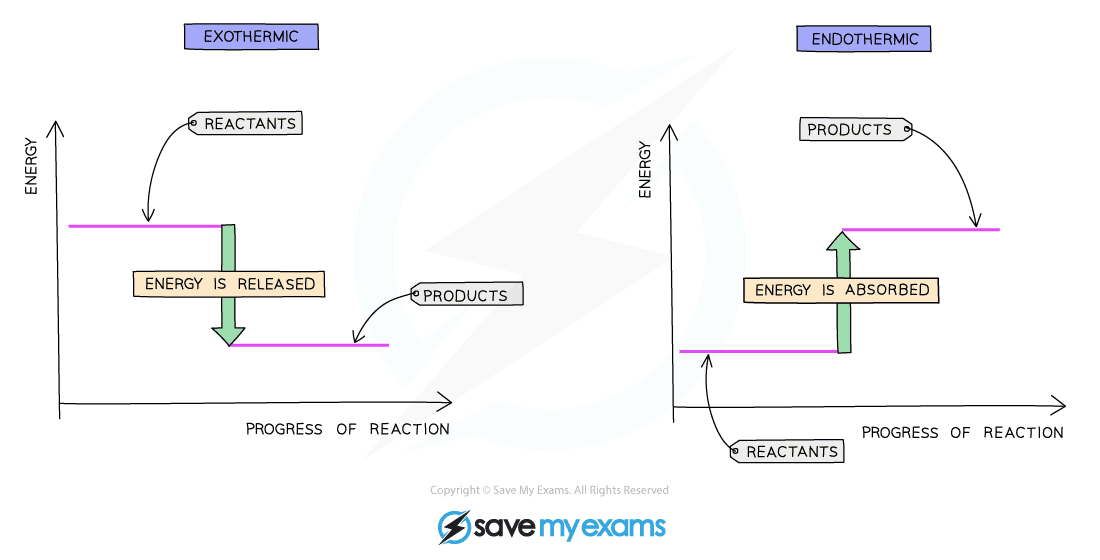
Diagrams showing the enthalpy change during an exothermic and endothermic reaction
Exothermic:
- A reaction in which energy is given out to surroundings (temperature of environment increases)
Examples:
- Combustion of fuels
- Reaction of acids and metals
- Neutralisation reactions
Endothermic:
- A reaction in which energy is taken in from surroundings (temperature of environment decreases)
- The energy change is positive
Examples:
- Thermal decomposition of carbonates
- Electrolysis
- First stages of photosynthesis
Exam Tip
To help you remember whether a chemical system is exothermic or endothermic, in EXothermic reactions heat Exits the system and in ENdothermic reactions heat ENters the system.
Exothermic reactions always give off heat and they feel hot, whereas endothermic reactions take heat in and they feel cold.Extended Only
Bond Breaking & Bond Forming
Endothermic and exothermic reactions
- Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break bonds and the energy released when the new bonds are formed
Endothermic
- If more energy is absorbed than is released, this reaction is endothermic
- More energy is required to break the bonds than that gained from making the new bonds
- The change in energy is positive since the products have more energy than the reactants
- The symbol ΔH (delta H) is used to show the change in heat energy. H is the symbol for enthalpy, which is a measure of the total heat of reaction of a chemical reaction
- Therefore an endothermic reaction has a positive ΔH value
Breaking chemical bonds requires energy which is taken in from the surroundings in the form of heat
Exothermic
- If more energy is released than is absorbed, then the reaction is exothermic
- More energy is released when new bonds are formed than energy required to break the bonds in the reactants
- The change in energy is negative since the products have less energy than the reactants
- Therefore an exothermic reaction has a negative ΔH value
Making new chemical bonds releases energy which radiates outwards from the reaction to the surroundings in the form of heat
Energy level diagrams
- These are graphical representations of the heat changes in chemical reactions
- The enthalpy of the reactants and products is displayed on the y-axis
- The reaction pathway is shown on the x-axis
- Arrows on the diagrams indicate whether the reaction is exothermic (downwards pointing) or endothermic (upwards pointing)
Diagrams showing the enthalpy change during an exothermic and endothermic reaction
Exothermic:
- During an exothermic reaction, energy is given out
- This means that the energy of the products will be lower than the energy of the reactants, so the change in enthalpy (ΔH) is negative
- This is represented on the energy-level diagram above with a downwards arrow as the energy of the products is lower than the reactants
Endothermic:
- During an endothermic reaction, energy is absorbed
- This means that the energy of the products will be higher than the energy of the reactants, so the change in enthalpy (ΔH) is positive
- This is represented on the energy-level diagram above with an upwards arrow as the energy of the products is higher than the reactants
Extended Only
Calculating the Energy of a Reaction
Energy of reaction calculations
- Each chemical bond has a specific bond energy associated with it
- This is the amount of energy required to break the bond or the amount of energy given out when the bond is formed
- This energy can be used to calculate how much heat would be released or absorbed in a reaction
- To do this it is necessary to know the bonds present in both the reactants and products
Method
- Add together all the bond energies for all the bonds in the reactants – this is the ‘energy in’
- Add together the bond energies for all the bonds in the products – this is the ‘energy out’
- Calculate the energy change: Energy change = energy in – energy out
Equation
Energy change = Energy taken in - Energy given out
Example: An exothermic reaction
Hydrogen and chlorine react to form hydrogen chloride gas:
H2 + Cl2 → 2HCl
The table below shows the bond energies relevant to this reaction:
Energy in = 436 + 243 = 679 KJ / Mole
Energy out = 2 x 432 = 864 KJ / Mole
Energy change = 679 – 864 = -185 KJ / Mole
*The energy change is negative, showing that energy is released to the surroundings so it is an exothermic reaction.
Example: An endothermic reaction
Hydrogen Bromide decomposes to form Hydrogen and Bromine:
2 x ( H - Br ) → H - H + Br - Br
The table below shows the bond energies relevant to this reaction:
Energy in = 2 x 366 = 732 KJ / Mole
Energy out = 436 + 193 = 629 KJ / Mole
Energy change = 732 – 629 = +103 KJ / Mole
*The energy change is positive, showing that energy is taken in from the surroundings, so is an endothermic reaction.
Exam Tip
For bond enthalpy questions, it is helpful to write down a displayed formula equation for the reaction before identifying the type and number of bonds, to avoid making mistakes.
The reaction thus becomes: H-H + Cl-Cl → H-Cl + H-Cl