Fuel, Combustion, Hydrogen
Energy from fuels
- A fuel is a substance which releases energy when burned
- When the fuel is a hydrocarbon then water and carbon dioxide are produced in combustion reactions
- Propane for example undergoes combustion according to the following equation:
C3H8 + 5O2 → 3CO2 + 4H2O ΔH = -2219 kJ/mol
- The efficiency of a fuel refers to how much energy is released per unit amount
- We can measure the efficiency of fuels by calorimetry
- A known mass of the fuel is combusted and used to heat up a known mass of water to calculate its heat of combustion
- Different fuels heat the water by different amounts and they can be analysed and compared in this way
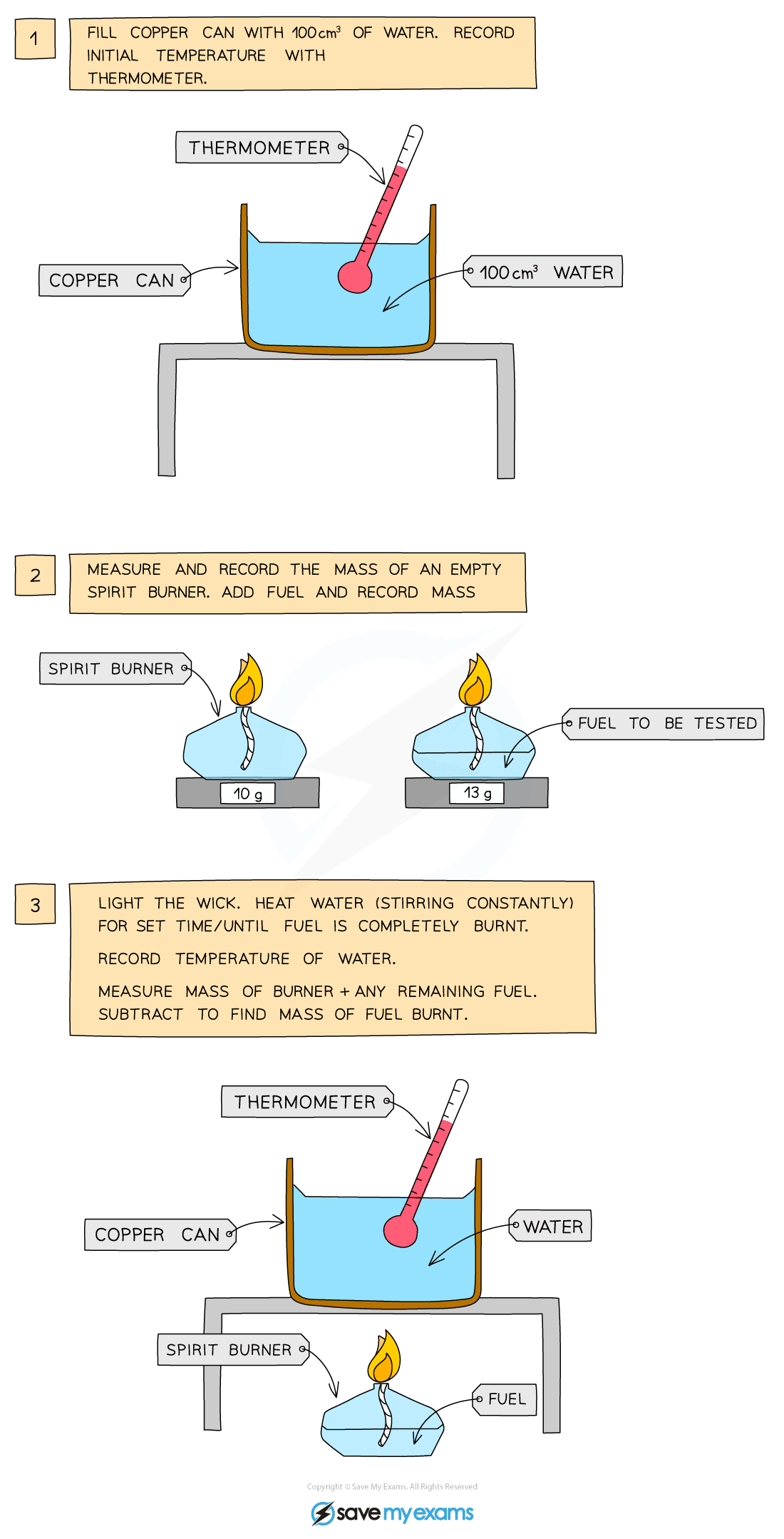
Calorimetry experiment – combustion
Diagram showing the calorimetry experiment for combustion
Method:
- Using a measuring cylinder, place 100 cm3 of water into a copper can
- Measure and record the initial temperature of the water
- Fill the spirit burner with test substance and measure and record its mass
- Place the burner under the copper can and light the wick
- Stir the water constantly with the thermometer and continue heating until the spirit burner burns out
- Measure and record the highest temperature of the water
Calculation:
Temperature change of water = final temperature – initial temperature
Number of moles burned = change in mass ÷ molecular mass
Amount of energy = change in temperature x mass of water x specific heat capacity
Amount of energy per mole (J mol-1) = total amount of energy ÷ moles burned
Hydrogen as a fuel
- Hydrogen is used in rocket engines and in fuel cells to power some cars
- Hydrogen has a series of advantages and disadvantages regarding its use as a fuel
- Advantages:
- It releases more energy per kilogram than any other fuel (except for nuclear fuels)
- It does not pollute as it only produces water on combustion, no other product is formed
- Disadvantages:
- Expensive to produce and requires energy for the production process
- Difficult and dangerous to store and move around (usually stored as liquid hydrogen in highly pressurised containers)
Radioactive isotopes as fuels
- Uranium-235 undergoes decay and gives off heat energy which nuclear power stations harness
- The heat it produces is used to heat water to steam, which in turn is used to power turbines to generate electricity
- Nuclear fuel energy is clean as it does not produce pollutants such as CO2 or oxides of nitrogen or sulfur
- But nuclear power plants are expensive to build and maintain as well as being potentially dangerous in the event of an accident as radioactive materials may be released
The nuclear fission of a large nucleus of uranium-235 into smaller daughter nucleiExtended Only
Fuel Cells
The hydrogen fuel cell
- A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode
- These cells are becoming more common in the automotive industry to replace petrol or diesel engines
- H2 and O2 are pumped through two porous electrodes where the half-reactions occur
- The following reaction occurs at the anode:
2H2 → 4H+ + 4e-
- At the cathode the following reaction takes place:
4H+ + O2 + 4e- → 2H2O
- The overall reaction is:
2H2 + O2 → 2H2O
- The electrons move around the external circuit from the cathode to the anode
- This movement of electrons is used to drive an electric motor
Diagram showing the movement of hydrogen, oxygen and electrons in a Hydrogen-Oxygen fuel cell
Advantages and disadvantages of fuel cells
- Advantages
- They do not produce any pollution
- They produce more energy per kilogram than either petrol or diesel
- No power is lost on transmission as there are far fewer moving parts than in an internal combustion engine
- Disadvantages
- Materials used in producing fuel cells are expensive
- High-pressure tanks are needed to store the oxygen and hydrogen in sufficient amounts
- Fuel cells are affected by low temperatures, becoming less efficient
- Hydrogen is expensive to produce and store