Obtaining Metals from their Ores
Extraction of ores from the Earth’s crust
- The Earth’s crust contains metals and metal compounds such as gold, iron oxide and aluminium oxide
- When found in the Earth, these are often mixed with other substances
- To be useful, the metals have to be extracted from their ores through processes such as electrolysis, using a blast furnace or by reacting with more reactive material
- The extraction of metals is a reduction process
- Unreactive metals do not have to be extracted as they are often found as the uncombined element as they do not easily react with other substances
Extraction of metal and the reactivity series
- The position of the metal on the reactivity series influences the method of extraction
- Those metals placed higher up on the series (above carbon) have to be extracted using electrolysis
- Metals lower down on the series can be extracted by heating with carbon
The reactivity series and extraction of metals
Extraction of Iron from Hematite
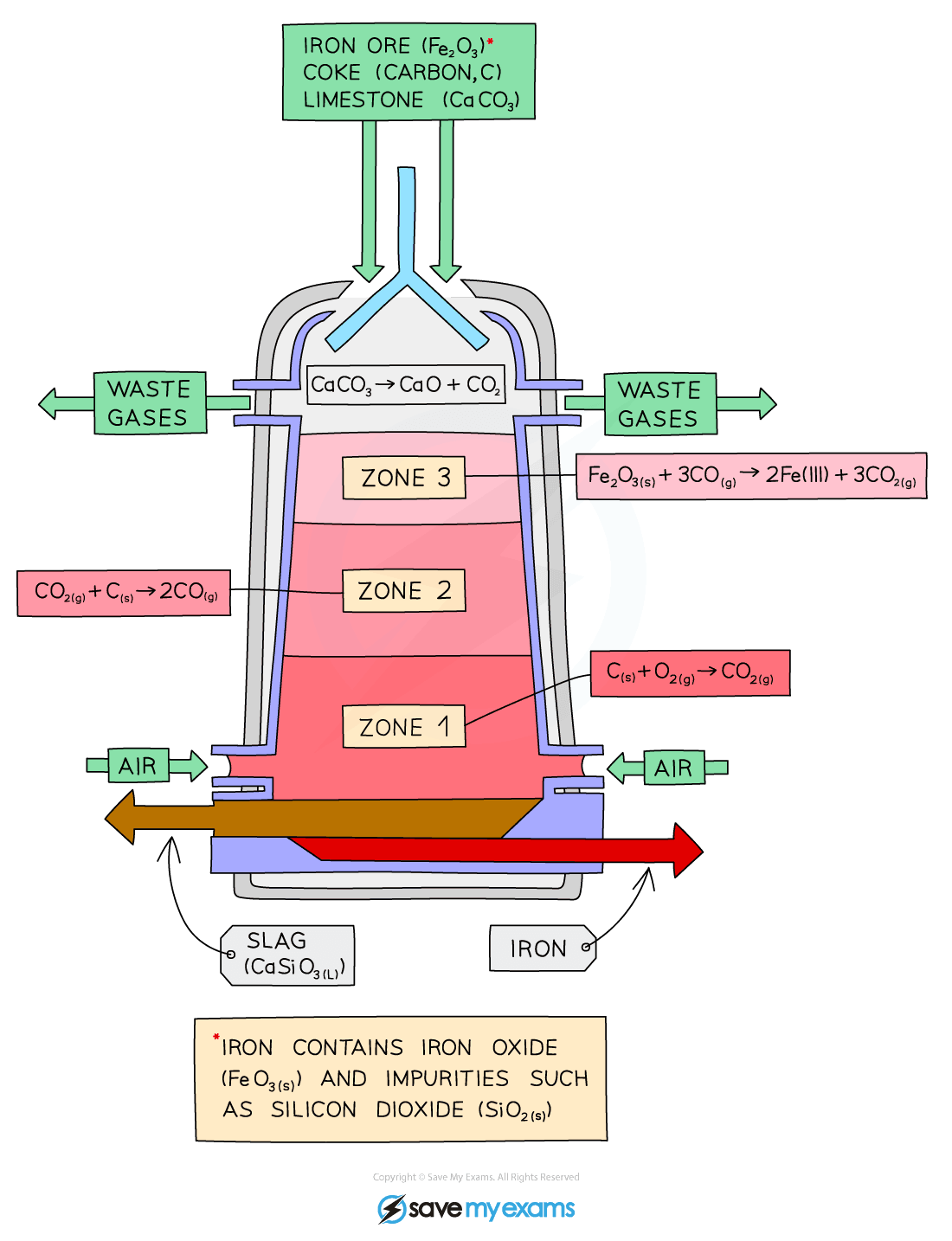
The extraction of iron in the blast furnace
Diagram showing the carbon extraction of iron
Raw Materials: Iron Ore (Haematite), Coke, Limestone and Air
Explanation:
- Iron Ore, Coke and Limestone are mixed together and fed into the top of the blast furnace. Hot air is blasted into the bottom of the blast furnace
- Zone 1
- Coke is used as the starting material.
- It is an impure carbon and it burns in the hot air blast to form carbon dioxide.
- This is a strongly exothermic reaction:
C (s) + O2 (g) → CO2 (g)
- Zone 2
- At the high temperatures in the furnace, carbon dioxide reacts with coke to form carbon monoxide:
CO2 (g) + C (s) → 2CO (g)
- Zone 3
- Carbon Monoxide (the reducing agent) reduces the Iron (III) Oxide in the Iron Ore to form Iron
- This will melt and collect at the bottom of the furnace, where it is tapped off:
Fe2O3 (s) + 3CO (g) → 2Fe (IIl) + 3CO2 (g)
- Limestone is added to the furnace to remove impurities in the ore.
- The Calcium Carbonate in the limestone decomposes to form calcium Oxide:
CaCO3 (s) → CaO (s) + CO2 (g)
- The Calcium Oxide reacts with the Silicon Dioxide, which is an impurity in the Iron Ore, to form Calcium Silicate
- This melts and collects as a molten slag floating on top of the molten Iron, which is tapped off separately:
CaO (s) + SiO2 (s) → CaSiO3 (l)
The Conversion of Iron into Steel
Making steel from iron
- Molten iron is an alloy of 96% iron, with carbon, phosphorus, silicon and sulfur impurities
- It is too brittle for most uses, so most of it is converted into steel by removing some of the impurities
- Not all of the carbon is removed as steel contains some carbon, the percentage of which depends on the use of the steel
- The molten iron is transferred to a tilting furnace where the conversion to steel takes place
- Oxygen and powdered calcium oxide are added to the iron
- The oxygen oxidises the carbon, phosphorus, silicon and sulfur to their oxides which are all acidic
- CO2 and SO2 are gaseous so escape from the furnace
- The acidic silicon and phosphorus oxides react with the powdered calcium oxide and from a slag which is mainly calcium silicate:
SiO2(l) + CaO(s) → CaSiO3(s)
- The slag floats on the surface of the molten iron and is removed.
Steel alloys
- The amount of carbon removed depends on the amount of oxygen used
- By carefully controlling the amount of carbon removed and subsequent addition of other metals such as chromium, manganese or nickel, the particular type of steel alloy is produced
Aluminium Extraction & Benefits of Recycling
Extraction of aluminium
- Aluminium is a reactive metal which sits above carbon on the reactivity series.
- It cannot be extracted from its ore (bauxite) by carbon reduction, so electrolysis is used.
Diagram showing the extraction of aluminium by electrolysis
Recycling metals: iron, steel and aluminium
Advantages
- Raw materials are conserved (bauxite and haematite)
- Energy use is reduced, especially in the electrolysis of aluminium
- Less pollution is produced as both processes contribute to air pollution
Disadvantages
- More transport on roads carrying used metals to recycling centres
- Energy consumed in collecting materials and sorting them per material type
Extended Only
The Process of Aluminium Extraction by Electrolysis
Raw materials: Aluminium Ore (Bauxite)
Explanation:
- The Bauxite is first purified to produce Aluminium Oxide Al2O3
- Aluminium Oxide has a very high melting point so it is first dissolved in molten Cryolite producing an electrolyte with a lower melting point, as well as a better conductor of electricity than molten aluminium oxide. This also reduces expense considerably
- The electrolyte is a solution of aluminium oxide in molten cryolite at a temperature of about 1000 °C. The molten aluminium is siphoned off from time to time and fresh aluminium oxide is added to the cell. The cell operates at 5-6 volts and with a current of 100,000 amps. The heat generated by the huge current keeps the electrolyte molten
- A lot of electricity is required for this process of extraction, this is a major expense
Reaction at the negative electrode:
The Aluminium melts and collects at the bottom of the cell and is then tapped off:
Al3+ + 3e- → Al
Reaction at the positive electrode:
2O2- - 4e- → O2
Some of the Oxygen produced at the positive electrode then reacts with the Graphite (Carbon) electrode to produce Carbon Dioxide Gas:
C (s) + O2 (g) → CO2 (g)
*This causes the carbon anodes to burn away, so they must be replaced regularly.Extended Only
The Process of Zinc Extraction
- Zinc ore is called zinc blende, ZnS
- The zinc blende is first converted to zinc oxide by heating with air:
2ZnS + 3O2 → 2ZnO + 2SO2
- The reducing agent is carbon monoxide which is formed inside the furnace through a series of reactions
- Carbon burns in a blast of very hot air to form carbon dioxide:
C + O2 → CO2
- The carbon dioxide produced reacts with more coke to form carbon monoxide:
CO2 + C → 2CO
- The carbon monoxide is the reducing agent and reduces the zinc oxide to zinc:
ZnO(s) + CO(g) →Zn(g) + CO2(g)
- Note that the zinc produced is in the gaseous state
- This passes out of the furnace and is cooled and condensed in a tray placed at the top of the furnace
- This is a key difference between the extraction of iron and aluminium, both of which are collected at the bottom of the furnace / electrolytic cell in the liquid state
Uses of zinc
- Zinc is used in galvanising, the process of coating a metal such as iron or steel with a protective coating of zinc to prevent corrosion or rusting
- Galvanising is an effective way of rust protection as it works even if the zinc coating becomes scratched or damaged
- The process can be done electrolytically or by dipping the metal parts into baths of molten zinc
- Zinc is also used to make an alloy called brass
- Brass contains 70% copper and 30% zinc
- The addition of zinc makes the alloy much harder and more corrosion resistant than copper alone