Factors Affecting Enzyme Action: Temperature
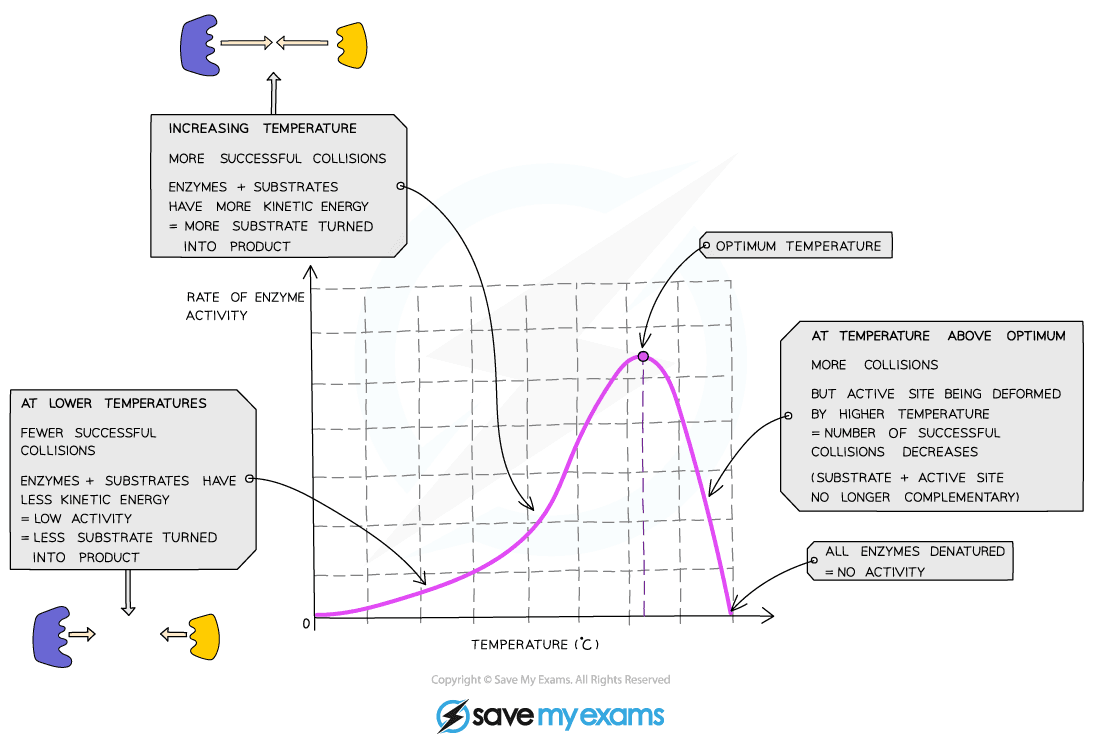
- Enzymes work fastest at their ‘optimum temperature’
- In the human body, this optimum temperature is about 37⁰C
- Heating to high temperatures (beyond the optimum) will break the bonds that hold the enzyme together and the active site will lose its shape
- The enzyme has been denatured
The effect of temperature on enzyme activity
- As temperature increases (towards the optimum) the activity of enzymes increases
- This is because the molecules have more kinetic energy, move faster and have more successful collisions with the substrate molecules. This leads to a faster rate of reaction
- This means that low temperatures do not denature enzymes, they just make them work more slowly due to a lack of kinetic energy
Graph showing the effect of temperature on the rate of enzyme activity
Factors Affecting Enzyme Action: pH
- The optimum pH for most human enzymes is pH 7
- Some enzymes that are produced in acidic conditions, such as the stomach, have a lower optimum pH (pH 2)
- Some that are produced in alkaline conditions, such as the duodenum, have a higher optimum pH (pH 8 or 9)
- If the pH is too far above or too far below the optimum, the bonds that hold the amino acid chain together to make up the protein can be disrupted or broken
- This will change the shape of the active site, so the substrate can no longer fit into it, reducing the rate of activity
- Moving too far away from the optimum pH will cause the enzyme to denature and the reaction it is catalysing will stop
Effect of pH on enzyme activity

Graph showing the effect of pH on the rate of activity for an enzyme from the duodenum
Factors Affecting Enzyme Action: Substrate Concentration
- The greater the substrate concentration, the greater the enzyme activity and the higher the rate of reaction:
- As the number of substrate molecules increases, the likelihood of enzyme-substrate complex formation increases
- If the enzyme concentration remains fixed but the amount of substrate is increased past a certain point, however, all available active sites eventually become saturated and any further increase in substrate concentration will not increase the reaction rate
- When the active sites of the enzymes are all full, any substrate molecules that are added have nowhere to bind in order to form an enzyme-substrate complex
- For this reason, in the graph below there is a linear increase in reaction rate as substrate is added, which then plateaus when all active sites become occupied
- At this point (known as the saturation point), the substrate molecules are effectively ‘queuing up’ for an active site to become available
The effect of substrate concentration on the rate of an enzyme-catalysed reaction
Exam Tip
Remember the terminology when writing about enzymes is very important. Make sure you refer to an enzyme becoming ‘denatured’ not ‘dying’.
Being able to describe AND explain the effect of each environmental condition on enzyme action is key.
Practise describing and explaining using the graphs and then check your descriptions against your notes.