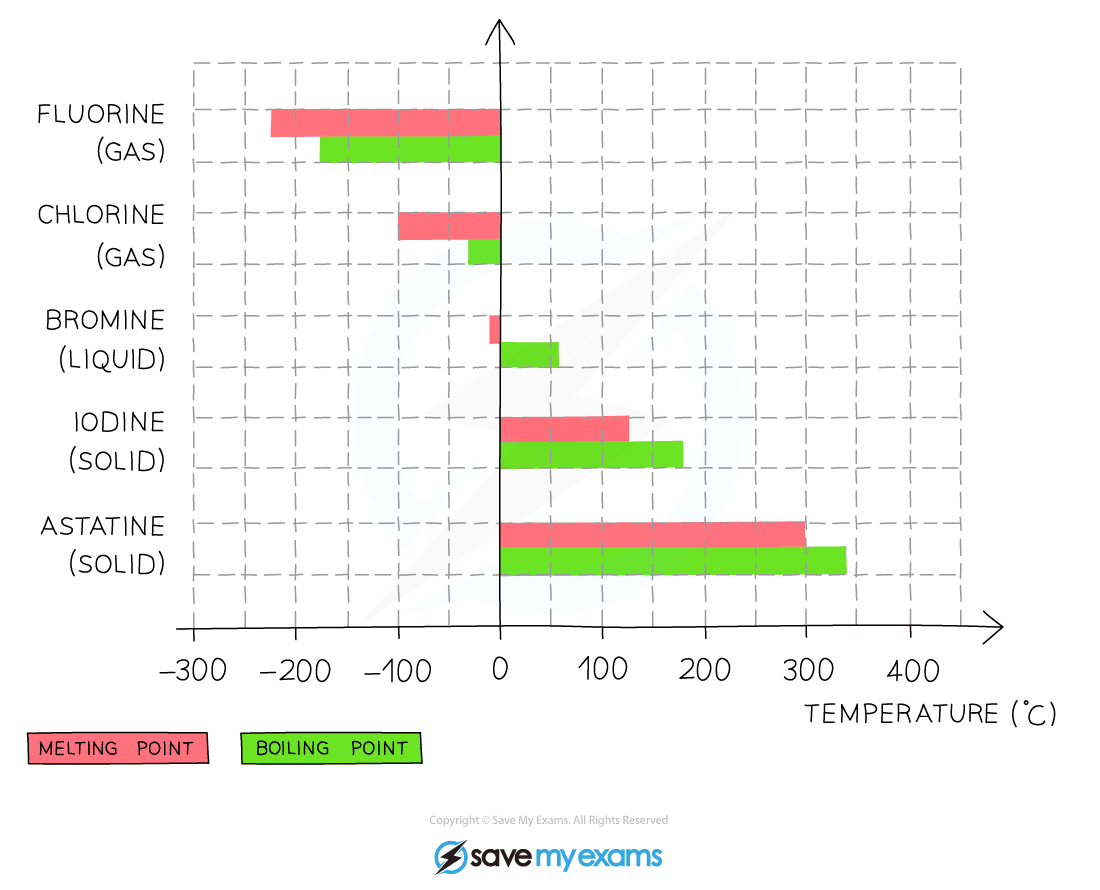Group I Properties & Trends: Basics
The Group I metals
- The Group I metals are also called the alkali metals as they form alkaline solutions with high pH values when reacted with water
- Group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium
- They all contain just one electron in their outer shell
Physical properties of the Group I metals
- The Group I metals:
- Are soft and easy to cut, getting softer and denser as you move down the Group (sodium and potassium do not follow the trend in density)
- Have shiny silvery surfaces when freshly cut
- Conduct heat and electricity
- They all have low melting points and low densities and the melting point decreases as you move down the Group
The alkali metals lie on the far left-hand side of the Periodic table
Chemical properties of the Group I metals
- They react readily with oxygen and water vapour in air so they are usually kept under oil to stop them from reacting
- Group 1 metals will react similarly with water, reacting vigorously to produce an alkaline metal hydroxide solution and hydrogen gas
Reactions of the Group I metals and water
Extended Only
Group I Properties & Trends
Electronic configuration and reactivity in Group I
Dot and cross diagram showing the electronic configuration of the first three elements in Group I
Explaining the trend in reactivity in Group I
- The reactivity of the Group 1 metals increases as you go down the group
- Each outer shell contains only one electron so when they react, they lose the outer electron which empties the outermost shell
- The next shell down automatically becomes the outermost shell and is already full, hence the atom obtains an electronic configuration which has a full outer shell of electrons
- As you go down Group 1, the number of shells of electrons increases by 1 (Period number increases down the Periodic table)
- This means that the outer electron is further away from the nucleus so there are weaker electrostatic forces of attraction
- This requires less energy to overcome the electrostatic forces of attraction between the negatively charged electron and the positively charged nucleus
- This allows the electron to be lost easily, making it more reactive as you go down the Group
Properties of other Alkali Metals (Rubidium, Caesium and Francium)
- As the reactivity of alkali metals increases down the Group, rubidium, caesium and francium will react more vigorously with air and water
- Lithium will be the least reactive at the top and francium will be the most reactive at the bottom
- Francium is rare and radioactive so is difficult to confirm predictions
Predicted reaction with Water
Group VII Properties & Trends
The halogens
- These are the Group 7 non-metals that are poisonous and include fluorine, chlorine, bromine, iodine and astatine
- Halogens are diatomic, meaning they form molecules of two atoms
- All halogens have seven electrons in their outer shell
- They form halide ions by gaining one more electron to complete their outer shells
Colours and states at room temperature
Trends in physical properties of the halogens
Melting point
- The density and melting and boiling points of the halogens increase as you go down the Group
Graph showing the melting and boiling points of halogens
State at room temperature
- At room temperature (20 °C), the physical state of the halogens changes as you go down the Group
- Chlorine is a gas, bromine is a liquid and iodine is a solid
The physical state of the halogens at room temperature
Colour
- The halogens become darker as you go down the group
- Chlorine is pale green, bromine is red-brown and iodine is black
The colours of the halogens
Electronic configuration and reactivity in Group VII
Dot and cross diagram showing the electronic configuration of the first three elements in Group VII
Explaining the trend in reactivity in Group VII
- Reactivity of Group 7 non-metals increases as you go up the Group
- Each outer shell contains seven electrons and when they react, they will need to gain one outer electron to get a full outer shell of electrons
- As you go up Group 7, the number of shells of electrons decreases (Period number decreases moving up the Periodic Table)
- This means that the outer electrons are closer to the nucleus so there are stronger electrostatic forces of attraction that attract the extra electron needed
- This allows an electron to be attracted more readily, so the higher up the element is in Group 7 then the more reactive it is
Reaction of the halogens with halide ions in displacement reactions
- A halogen displacement reaction occurs when a more reactive halogen displaces a less reactive halogen from an aqueous solution of its halide
- The reactivity of Group 7 non-metals increases as you move up the Group
- Out of the 3 halogens, chlorine, bromine and iodine, chlorine is the most reactive and iodine is the least reactive
Aqueous solution colour of halogens
Halogen displacement reactions
Chlorine and bromine
- If you add chlorine solution to colourless potassium bromide solution, the solution becomes orange as bromine is formed
- Chlorine is above bromine in Group 7 so is more reactive
- Chlorine will therefore displace bromine from an aqueous solution of metal bromide
Potassium Bromide + Chlorine → Potassium Chloride + Bromine
2KBr (aq) + Cl2 (aq) → 2KCl (aq) + Br2(aq)
Bromine and iodine
- Bromine is above iodine in Group 7 so is more reactive
- Bromine will therefore displace iodine from an aqueous solution of metal iodide
Bromine + Magnesium Iodide → Magnesium Bromide + Iodine
Br2 (l) + 2MgI (aq) → 2MgBr (aq) + I2 (aq or s)
Exam Tip
Iodine solid, solution and vapour are different colours. Solid iodine is dark grey-black, iodine vapour is purple and aqueous iodine is brown.
Properties of the other Halogens (Fluorine & Astatine)
Melting and boiling point
- The melting and boiling point of the halogens increases as you go down the Group
- Fluorine is at the top of Group 7 so will have the lowest melting and boiling point
- Astatine is at the bottom of Group 7 so will have the highest melting and boiling point
Physical states
- The halogens become harder as you go down the Group
- Fluorine is at the top of Group 7 so will be a gas
- Astatine is at the bottom of Group 7 so will be a solid
Colour
- The colour of the halogens becomes darker as you go down the Group
- Fluorine is at the top of Group 7 so the colour will be lighter, so fluorine is yellow
- Astatine is at the bottom of Group 7 so the colour will be darker, so astatine is black