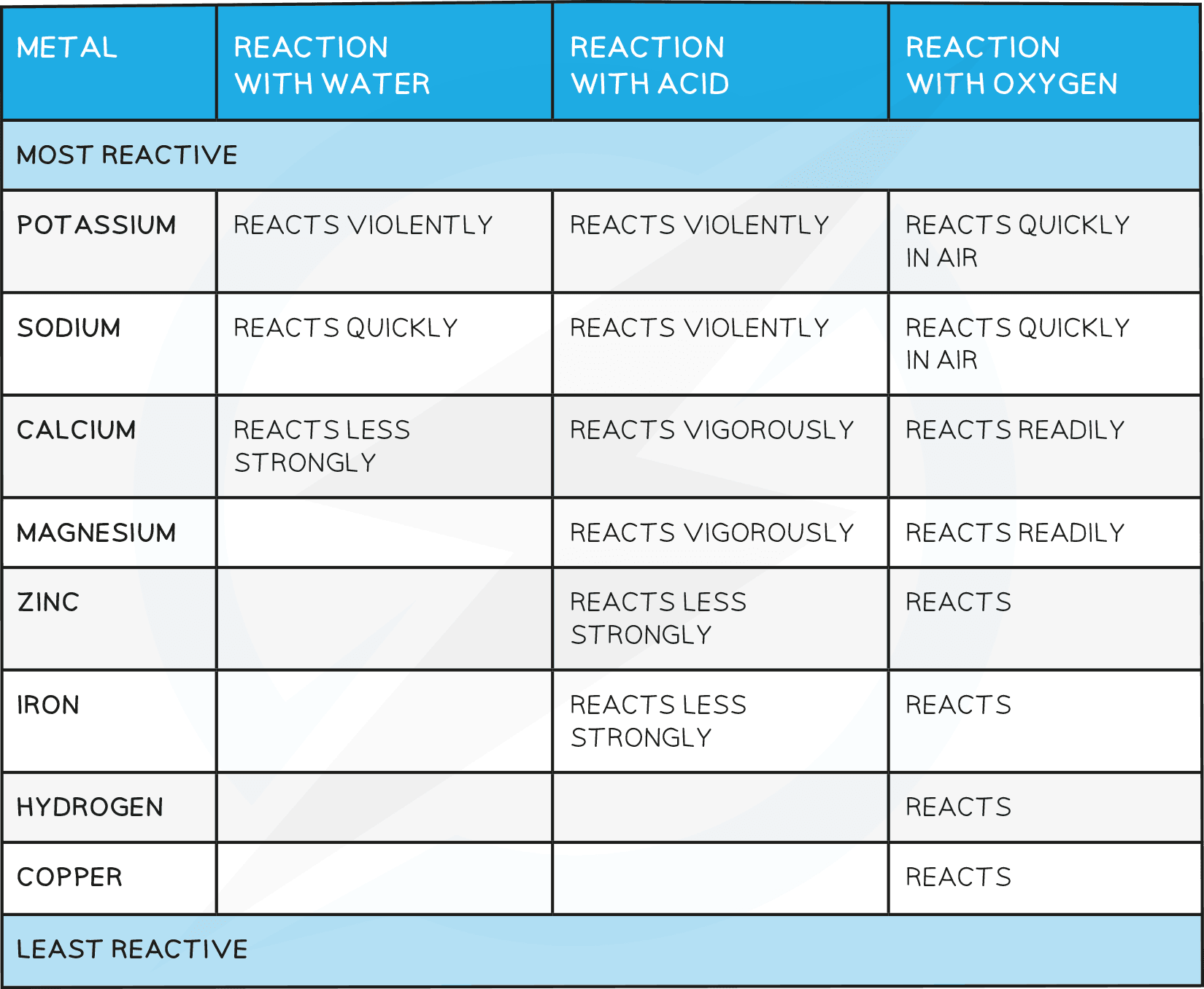
The Reactivity Series
- The chemistry of the metals is studied by analysing their reactions with water, dilute acid and oxygen
- Based on these reactions a reactivity series of metals can be produced
- The series can be used to place a group of metals in order of reactivity based on the observations of their reactions with water, acid and oxygen
Carbon and the reactivity series mnemonic
- Carbon is an important element and has its own place on the reactivity series
- Its use in the extraction of metals from their oxides is discussed in this section but a more complete reactivity series with an accompanying mnemonic to help you memorise it is below
The reactivity series mnemonic
- “Please send lions, cats, monkeys and cute zebras into hot countries signed Gordon”
Extended Only
Reactions with Aqueous Ions & Oxides
- The reactivity of metals increases going up the reactivity series
- This means that a more reactive metal can displace a less reactive metal from its oxide by heating
Example: Copper(II) Oxide
- It is possible to reduce copper(II) oxide by heating it with magnesium
- As magnesium is above copper in the reactivity series, magnesium is more reactive so can displace copper
- The reducing agent in the reaction is magnesium:
CuO (s) + Mg (s) → Cu (s) + MgO (s)
Other common reactions
Displacement reactions between metals and aqueous solutions of metal salts
- Any metal will displace another metal that is below it in the reactivity series from a solution of one of its salts
- This is because more reactive metals lose electrons and form ions more readily than less reactive metals, making them better reducing agents
- The less reactive metal is a better electron acceptor than the more reactive metal, thus the less reactive metal is reduced. (OIL-RIG: reduction is gain of electrons)
Example: Zinc and copper(II) sulfate
- As Zinc is above copper in the reactivity series, zinc is more reactive so can displace copper from copper(II) sulfate solution:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Other Common Reactions
Extended Only
Heating Metal Hydroxides, Carbonates & Nitrates
Thermal decomposition reactions
- Some compounds decompose or breakdown when they are heated to sufficiently high temperatures
- These reactions are called thermal decomposition reactions
- A common example is the thermal decomposition of calcium carbonate (limestone), which occurs at temperatures above 800ºC:
CaCO3 → CaO + CO2
Thermal decomposition of metal hydroxides
- Most metal hydroxides undergo thermal decomposition
- Water and the corresponding metal oxide are the products formed, for example zinc hydroxide thermally decomposes as follows:
Zn(OH)2 → ZnO + H2O
- Group II metal hydroxides decompose similarly but the Group I hydroxides (apart from lithium) do not decompose due to their having a higher thermal stability
Thermal decomposition of metal carbonates
- Most of the metal carbonates and hydrogen carbonates undergo thermal decomposition
- The metal oxide and carbon dioxide are the products formed, for example magnesium carbonate thermally decomposes as follows:
MgCO3 → MgO + CO2
- Group I carbonates (again apart from lithium carbonate) do not decompose when heated
- This is due to the high thermal stability of reactive metals; the more reactive the metal then the more difficult it is to decompose its carbonate
- CuCO3 for example is relatively easy to thermally decompose but K2CO3 does not decompose
Thermal decomposition of metal nitrates
- All of the metal nitrates decompose when they are heated
- Group I nitrates decompose forming the metal nitrite and oxygen, for example sodium nitrate decomposes as follows:
2NaNO3 → 2NaNO2 + O2
- Most other metal nitrates form the corresponding metal oxide, nitrogen dioxide and oxygen when heated, for example copper nitrate:
2Cu(NO3)2→ 2CuO + 4NO2 + O2
Aluminium and its apparent lack of reactivity
- Aluminium is a curious metal in terms of its reactivity
- It is placed high on the reactivity series but it doesn´t react with water or acids
- This is because the surface of aluminium metal reacts with oxygen in the air forming a protective coating of aluminium oxide:
4Al + 3O2 → 2Al2O3
- The aluminium oxide layer is tough, unreactive and resistant to corrosion
- It adheres very strongly to the aluminium surface and protects it from reaction with other substances, hence making it appear unreactive
Exam Tip
For the thermal decomposition reactions, you will need to be able to describe how the Group I nitrates differ from the other metals.
You should be able to write out the balanced symbol equations for these reactions.