Classifying Oxides
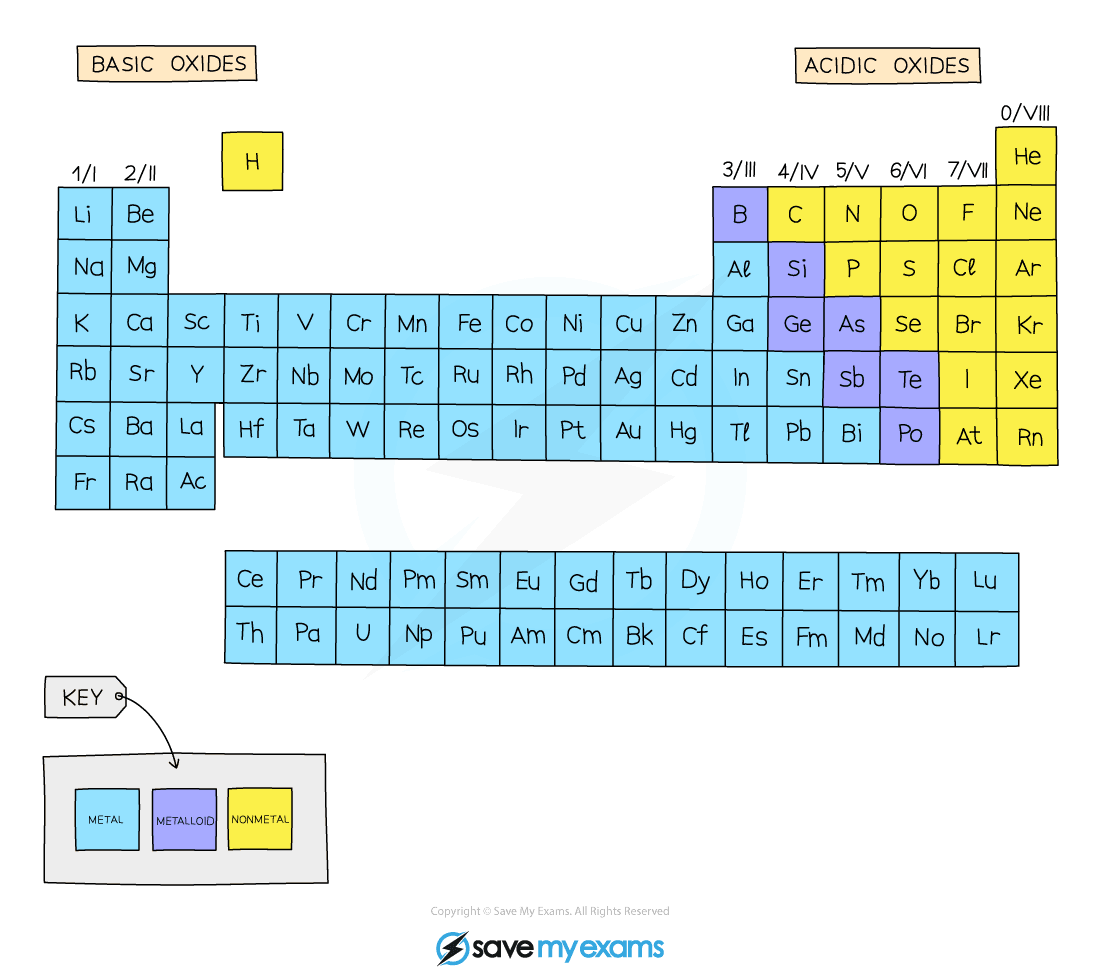
Acid and basic oxides
- Acidic and basic oxides have different properties and values of pH
- The difference in their pH stems from whether they are bonded to a metal or a nonmetal element
- The metallic character of the element influences the acidic or alkaline behaviour of the molecule
Metals form basic oxides and hydroxides while nonmetals form acidic oxides
Acidic oxides
- Acidic oxides are formed when a nonmetal element combines with oxygen
- They react with bases to form a salt and water
- When dissolved in water they produce an acidic solution with a low pH
- Common examples include SO2 and SiO2
Basic oxides
- Basic oxides are formed when a metal element combines with oxygen
- They react with acids to form a salt and water
- When dissolved in water they produce a basic solution with a high pH
- Common examples include NaOH, KOH and Ca(OH)2
Extended Only
Neutral & Amphoteric Oxides
Neutral oxides
- Some oxides do not react with either acids or bases and thus are said to be neutral
- Examples include N2O, NO and CO
Amphoteric oxides
- Amphoteric oxides are a curious group of oxides that can behave as both acidic and basic, depending on whether the other reactant is an acid or a base
- In both cases a salt and water is formed
- Two most common amphoteric oxides are zinc oxide and aluminum oxide
- The hydroxides of both of these elements also behave amphoterically
- Example of aluminium oxide behaving as a base:
Al2O3 + 6HCl → 2AlCl3 + 3H2O
- Example for an aluminium oxide behaving as an acid:
Al2O3 + 2NaOH → 2NaAlO2 + H2O