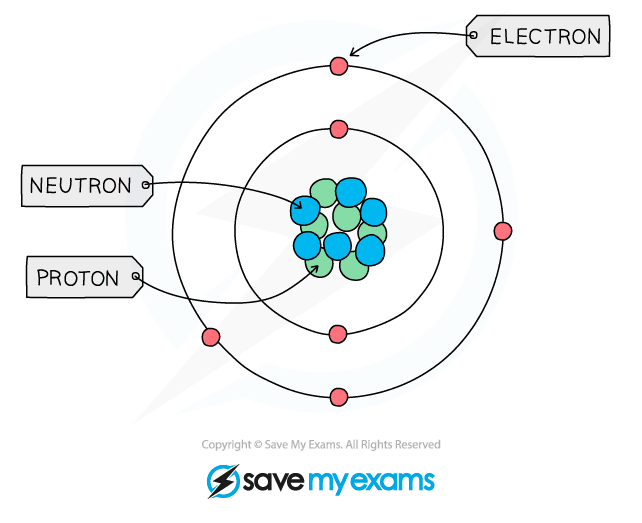
Protons, Neutrons & Electrons
- Elements are made of tiny particles of matter called atoms
- Each atom is made of subatomic particles called protons, neutrons and electrons
- Their size is so tiny that we can’t really compare their masses in conventional units such as kilograms or grams, so a unit called the relative atomic mass is used
- One relative atomic mass unit is equal to the mass of a carbon-12 atom.
- All other elements are measured relative to the mass of a carbon-12 atom and since these are ratios, the relative atomic mass has no units
- Hydrogen for example has a relative atomic mass of 1, meaning that 12 atoms of hydrogen would have exactly the same mass as 1 atom of carbon
- The relative mass and charge of the subatomic particles are shown below:
Defining Proton Number
- The atomic number (or proton number) is the number of protons in the nucleus of an atom. The symbol for this number is Z
- It is also the number of electrons present in an atom and determines the position of the element on the Periodic Table
Defining Nucleon Number
- Nucleon number (or mass number) is the total number of protons and neutrons in the nucleus of an atom. The symbol for this number is A
- The nucleon number minus the proton number gives you the number of neutrons of an atom
- Note that protons and neutrons can collectively be called nucleons.
- The atomic number and mass number for every element is on the Periodic Table
Diagram showing the notation used on the Periodic Table
Electrons (symbol e–)
- These subatomic particles move very fast around the nucleus
- They move in orbital paths called shells
- The mass of the electron is negligible, hence the mass of an atom is contained within the nucleus where the neutron and proton reside

The structure of the carbon atom

The symbol key for carbon as represented on the Periodic Table
Exam Tip
Both the atomic number and the mass number are given on the Periodic Table but it can be easy to confuse them.
Think MASS = MASSIVE, as the mass number is always the bigger of the two numbers, the other smaller one is thus the atomic / proton number.
The Basis of the Periodic Table
- Elements are arranged on the Periodic table in order of increasing atomic number where each element has one proton more than the element preceding it
- Hydrogen has 1 proton, helium has 2 protons, lithium has 3 etc.
- The table is arranged in vertical columns called Groups numbered I – VIII and in rows called Periods
- Elements in the same group have the same amount of electrons in their outer shell, which gives them similar chemical properties

The Periodic Table
Exam Tip
The proton number is unique to each element and could be considered as an element’s “fingerprint”. Electrons come and go during chemical processes but the proton number doesn’t change.
Defining Isotopes
- Isotopes are atoms of the same element that contain the same number of protons and electrons but a different number of neutrons.
- The symbol for an isotope is the chemical symbol (or word) followed by a dash and then the mass number.
- So C-14 is the isotope of carbon which contains 6 protons, 6 electrons and 14 – 6 = 8 neutrons.
The atomic structure and symbols of the three isotopes of hydrogen:
Types of Isotope
- Isotopes can be divided into two categories: radioactive and non-radioactive
- Radioactive isotopes (radioisotopes) are unstable due to the imbalance of neutrons and protons, which causes the nucleus to decay over time through nuclear fission and emit radiation. Examples of radioisotopes include tritium and carbon-14
- Decay occurs at a different rate for each isotope, but the time taken for the radioactivity of an isotope to decrease by 50% is constant for that particular isotope and is known as the half-life
- Radioactive isotopes have numerous medical and industrial uses
- Non-radioactive isotopes are stable atoms which really only differ in their mass
Uses of Radioactive Isotopes
Medical uses
- Radiation is extremely harmful and kills cells so isotopes are used to treat cancer. The isotope cobalt-60 is frequently used for this purpose
- Medical tracers as certain parts of the body absorb isotopes and others do not. In this way an isotope can be injected into the blood and its path through the body traced with a radioactive detecting camera, revealing the flow of blood through bodily systems
- Medical instruments and materials are routinely sterilized by exposure to radiation, which kills any bacteria present
Industrial uses
- Radioactive dating uses the carbon-14 isotope to date carbon-containing materials such as organic matter, rocks and other artefacts. The half-life of C-14 is 5730 years and so this technique is often used to date very old historical objects
- Similar to medical use, radioactive tracers are deployed to detect leaks in gas or oil pipes
- The radioactive isotope uranium-235 is used as nuclear in power plants in controlled fission reactions
Exam Tip
Radioactive decay is a random process which occurs inside the nucleus and is independent of temperature, pressure, pH etc. It is a nuclear process and is not considered a chemical reaction.Extended Only
Why Isotopes Share Properties
- Isotopes of the same element display the same chemical characteristics
- This is because they have the same number of electrons in their outer shells and this is what determines an atom’s chemistry
- The difference between isotopes is the neutrons which are neutral particles within the nucleus and add mass only
Electron Shells
Electronic structure
- We can represent the structure of the atom in two ways: using diagrams called electron shell diagrams or by writing out a special notation called the electronic configuration
Electron shell diagrams
- Electrons orbit the nucleus in shells (or energy levels) and each shell has a different amount of energy associated with it
- The further away from the nucleus then the more energy a shell has.
- Electrons occupy the shell closest to the nucleus which can hold only 2 electrons and which go in separately
- When a shell becomes full electrons then fill the next shell
- The second shell can hold 8 electrons and the third shell can also hold 8 electrons and the electrons organise themselves in pairs in these shells
- The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
The electron shells
Electronic configuration
- The arrangement of electrons in shells can also be explained using numbers
- There is a clear relationship between the outer shell electrons and how the Periodic Table is designed
- The number of notations in the electronic configuration will show the number of shells of electrons the atom has, showing the Period in which that element is in
- The last notation shows the number of outer electrons the atom has, showing the Group that element is in
- Elements in the same Group have the same number of outer shell electrons
The electronic configuration for chlorine
Period: The red numbers at the bottom show the number of notations which is 3, showing that a chlorine atom has 3 shells of electrons
Group: The green box highlights the last notation which is 7, showing that a chlorine atom has 7 outer electrons
The position of chlorine on the Periodic Table
The noble gases
- The atoms of the Group 8/0 elements all have 8 electrons in their outer shells, with the exception of helium which has 2. But since helium has only 2 electrons in total and thus the first shell is full (which is the only shell), it is thus the outer shell so helium also has a full valency shell
- All of the noble gases are unreactive as they have full outer shells and are thus very stable
- All elements wish to fill their outer shells with electrons as this is a much more stable and desirable configuration
The noble gases are on the Periodic Table in Group 8/0
The electronic configuration of the first twenty elements
Note: although the third shell can hold up to 18 electrons, the filling of the shells follows a more complicated pattern after potassium and calcium. For these two elements, the third shell holds 8 and the remaining electrons (for reasons of stability) occupy the fourth shell first before filling the third shell.