Electrical Conductivity & Malleability of Metals
- Metal atoms are held together strongly by metallic bonding
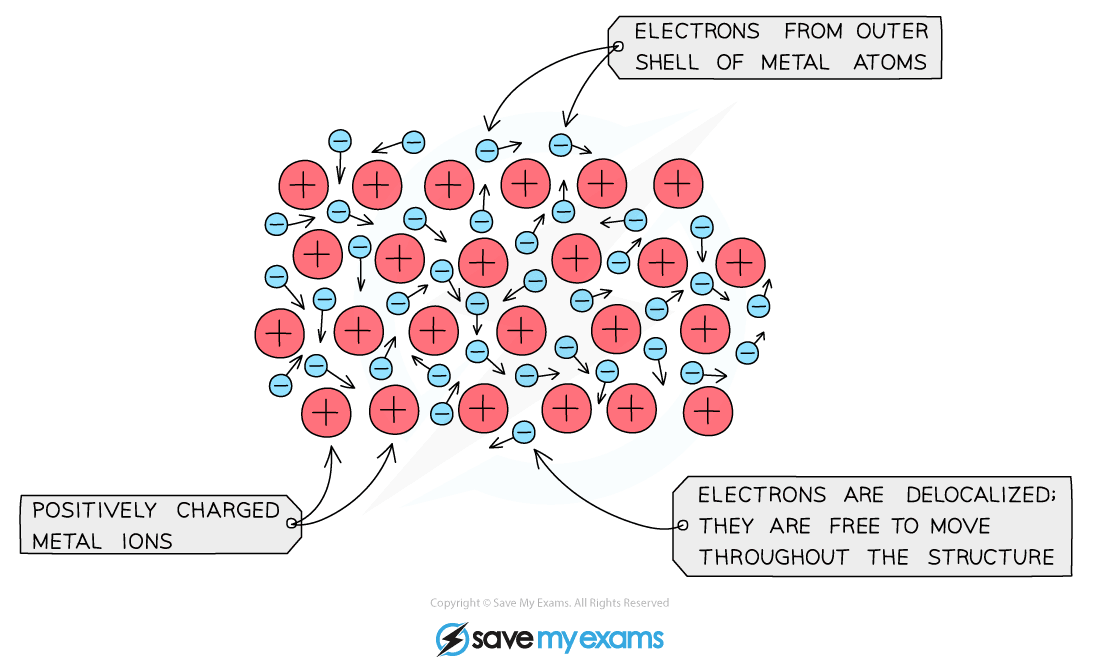
- Within the metal lattice, the atoms lose their valence electrons and become positively charged
- The valence electrons no longer belong to any metal atom and are said to be delocalised
- They move freely between the positive metal ions like a sea of electrons
- Metallic bonds are strong and are a result of the attraction between the positive metal ions and the negatively charged delocalised electrons
Diagram showing metallic lattice structure with delocalised electrons
Link between metallic bonding and the properties of metals
- Metals have high melting and boiling points
- There are many strong metallic bonds in giant metallic structures
- A lot of heat energy is needed to overcome forces and break these bonds
- Metals conduct electricity
- There are free electrons available to move and carry charge
- Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
- Hence electrons can flow so electricity is conducted
- Metals are malleable and ductile
- Layers of positive ions can slide over one another and take up different positions
- Metallic bonding is not disrupted as the valence electrons do not belong to any particular metal atom so the delocalised electrons will move with them
- Metallic bonds are thus not broken and as a result metals are strong but flexible
- They can be hammered and bent into different shapes without breaking