Electrolysis: Basics
- When an electric current is passed through a molten ionic compound the compound decomposes or breaks down
- The process also occurs for aqueous solutions of ionic compounds
- Covalent compounds cannot conduct electricity hence they do not undergo electrolysis
- Ionic compounds in the solid state cannot conduct electricity either since they have no free ions that can move and carry the charge
Particles in ionic compounds are in fixed position in the solid state but can move around when molten or in solution
Key terms
- Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte
- Electrolyte is the ionic compound in molten or dissolved solution that conducts the electricity
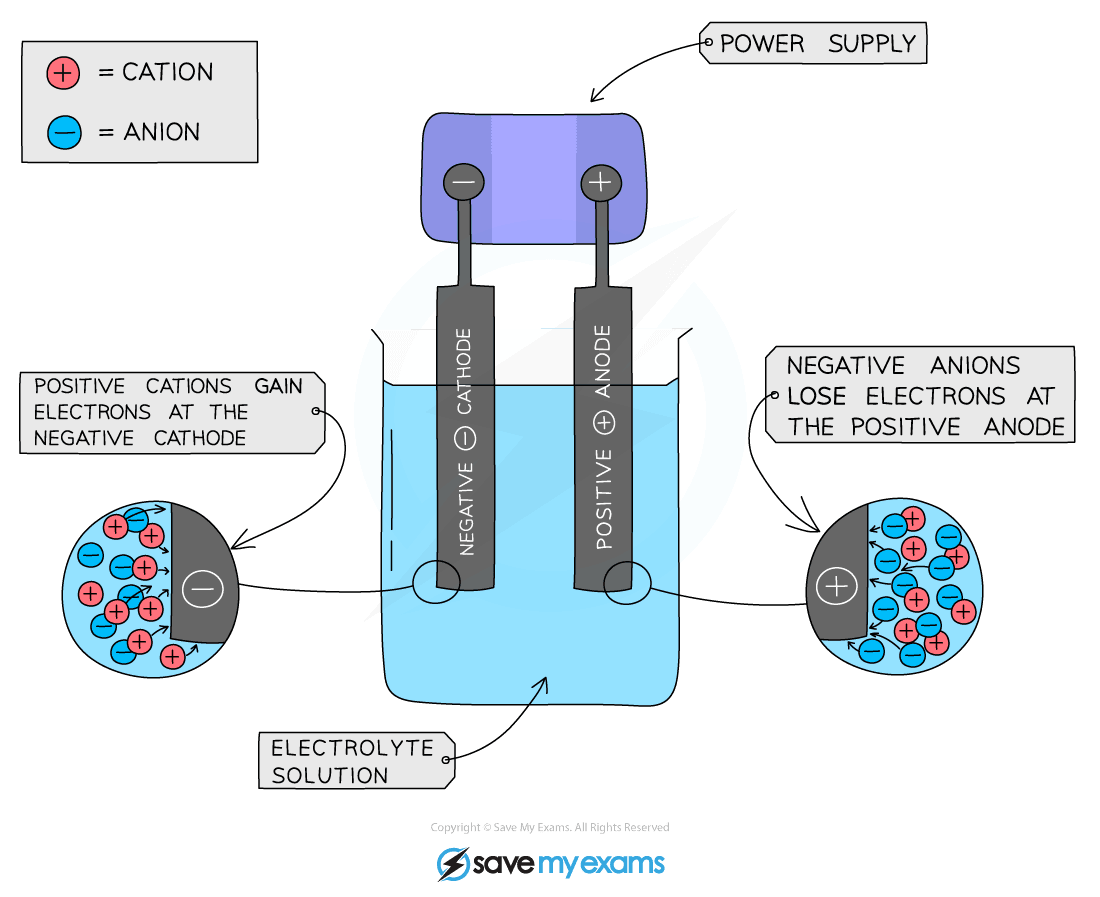
- Anode is the positive electrode of an electrolysis cell
- Anion is a negatively charged ion which is attracted to the anode
- Cathode is the negative electrode of an electrolysis cell
- Cation is a positively charged ion which is attracted to the cathode
The basic set-up of an electrolysis cell
Electrolysis of molten compounds eg: Lead (II) Bromide:
Diagram showing the electrolysis of lead (II) bromide
Method:
- Add lead (II) bromide into a beaker and heat so it will turn molten, allowing ions to be free to move and conduct an electric charge
- Add two graphite rods as the electrodes and connect this to a power pack or battery
- Turn on power pack or battery and allow electrolysis to take place
- Negative bromide ions move to the positive electrode (anode) and lose two electrons to form bromine molecules. There is bubbling at the anode as brown bromine gas is given off
- Positive lead ions move to the negative electrode (cathode) and gain electrons to form a grey lead metal which deposits on the surface of the electrode
Reaction at electrodes:
Exam Tip
Use the PANIC mnemonic to remember which electrode is the positive and which is the negative:
Positive (is) Anode Negative Is Cathode
Cations are attracted to the cathode and anions are attracted to the anode. Electron flow in electrochemistry occurs in alphabetical order as electrons flow from the Anode to the Cathode.
Electrolysis: General Principles
Rules
- Aqueous solutions will always have water (H2O)
- H+ and OH– ions from the water are involved as well
Positive electrode (anode)
- OH– ions and non-metal ions (anions) are attracted to the positive electrode
- Either OH– or non-metal ions will lose electrons and oxygen gas or gas of non-metal in question is released, eg. chlorine, bromine, nitrogen
- The product formed depends on which ion loses electrons more readily, with the more reactive ion remaining in solution
A reactivity series of anions is shown below:
More reactive SO42- → NO3- → Cl- → Br- → I-→ OH- Less reactive
Negative electrode (cathode)
- H+ and metal ions attracted to the negative electrode but only one will gain electrons
- Either hydrogen or metal will be produced
- If the metal is above hydrogen in reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
The reactivity series of metals including hydrogen and carbon
Concentrated and dilute solutions
- Concentrated and dilute solutions of the same compound give different products
- For anions, the more concentrated ion will tend to get discharged over a more dilute ion
Electrolysis of binary molten compound
- For a binary molten compound of a metal and a nonmetal, the cathode product will always be the metal
- The product formed at the anode will always be the non-metal
Electrolysis of Aqueous Solutions
Diagram showing the electrolysis of aqueous solutions
Method:
- Add aqueous solution into a beaker
- Add two Graphite rods as the electrodes and connect this to a power pack or battery
- Turn on power pack or battery and allow electrolysis to take place
Exam Tip
Use the PANIC mnemonic to remember which electrode is the positive and which is the negative: Positive (is) Anode Negative Is Cathode.
Cations are attracted to the cathode and anions are attracted to the anode. Electron flow in electrochemistry occurs in alphabetical order as electrons flow from the Anode to the Cathode.Extended Only
Electrolysis: Reactions at the Electrodes
Determining what gas is produced
- If the gas produced at the cathode burns with a ‘pop’ when a sample is lit with a lighted splint then the gas is hydrogen
- If the gas produced at the anode relights a glowing splint dipped into a sample of the gas then the gas is oxygen
- The halogen gases all produce their own colours (bromine is red-brown, chlorine is yellow-green and fluorine is pale yellow)
Extended Only
Products of Electrolysis & Charge Transfer
Copper refining
- The electrolysis of CuSO4 using graphite rods produces oxygen and copper
- By changing the electrodes from graphite to pure and impure copper, the products can be changed at each electrode
- Electrolysis can be used to purify metals by separating them from their impurities
- In the set-up, the impure metal is always the anode, in this case the impure copper
- The cathode is a thin sheet of pure copper
- The electrolyte used is an aqueous solution of a soluble salt of the pure metal at the anode, e.g: CuSO4
- Copper atoms at the anode lose electrons, go into solution as ions and are attracted to the cathode where they gain electrons and form now purified copper atoms
- The anode thus becomes thinner due to loss of atoms and the impurities fall to the bottom of the cell as sludge
- The cathode gradually becomes thicker
Electrolysis of halide solutions
- We have seen that cations lower down on the reactivity series tend to be discharged in preference to more reactive cations
- The same occurs for anions which can be arranged in order of ease of discharge:
More reactive SO42- → NO3- → OH- → Cl- → Br- → I- Less reactive
- Eg. in a concentrated aqueous solution of barium chloride, the Cl– ions are discharged more readily than the OH– ions, so chlorine gas is produced at the anode
- If the solution is dilute however only the OH– ion is discharged and so oxygen would be formed
Transfer of Charge
- During electrolysis the electrons move from the power supply towards the cathode
- Positive ions within the electrolyte move towards the negatively charged electrode which is the cathode
- Here they accept electrons from the cathode and either a metal or hydrogen gas is produced
- Negative ions within the electrolyte move towards the positively charged electrode which is the anode
- If the anode is inert (such as graphite or platinum), the ions lose electrons to the anode and form a nonmetal or oxygen gas
- If the anode is a reactive metal, then the metal atoms of the anode lose electrons and go into solution as ions, thinning the anode
Diagram showing the direction of movement of electrons and ions in the electrolysis of NaClExtended Only
Ionic Half-Equations & Electrical Energy
- Reduction occurs at the cathode as the positive ions gain electrons
Electrochemical cells
- An electrochemical cell is a source of electrical energy
- The simplest design consists of two electrodes made from metals of different reactivity immersed in an electrolyte and connected to an external circuit
- A common example is zinc and copper
- Zinc is the more reactive metal and forms ions more easily, releasing electrons as its atoms form ions
- The electrons give the more reactive electrode a negative charge and they then flow around the circuit to the copper electrode
- The difference in the ability of the electrodes to release electrons causes a voltage to be produced
- The greater the difference in the metal’s reactivity, the greater the voltage
Electrochemical cell made with copper and magnesium. These metals are further apart on the reactivity series than copper and zinc and would hence produce a greater voltage
Exam Tip
When a metal conducts it is the electrons that are moving through the metal. When a salt solution conducts it is the ions in the solution that move towards the electrodes carrying the electrons.
During electrolysis oxidation of the non-metal ions always occurs at the anode and reduction of the metal or hydrogen ions occurs at the cathode.